Describe the Difference Between Absorption and Adsorption
Adsorption chromatography separates compounds by adsorption while partition chromatography separates compounds by partition. 13 rows Absorption is when a substance is absorbed or taken by in bulk by another substance while.

Difference Between Absorption And Adsorption Compare The Difference Between Similar Terms
This is the key difference between adsorption chromatography and partition chromatography.
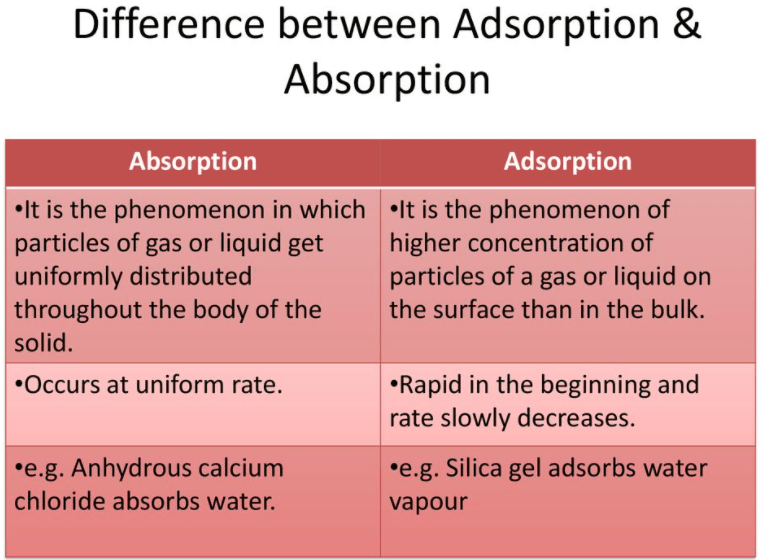
. The main difference between absorption and adsorption is that absorption is the process in which a fluid dissolves by a liquid or a solid. The reaction rate in absorption is endothermic and uniform while adsorption is exothermic and increases slowly to attain equilibrium. On the alternative adsorption is the strategy whereby ions molecules or atoms from a substance like gasoline robust or liquid adhere to flooring of adsorbent.
In absorption the heat exchange process is endothermic whereas in adsorption the heat exchange process is. Absorption is a bulk phenomenon whereas adsorption is a surface phenomenon. Refrigeration and Air Conditioning Technology 7th Edition Edit edition Solutions for Chapter 34 Problem 11RQ.
Absorption takes place at a uniform rate. Absorption is the incorporation of a material or substance in one state into another material or substance of a different state. Physical adsorption is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption.
In the case of Adsorption the atoms ions or molecules from a substance adhere to a surface of the adsorbent. However the term sorption covers both absorption and adsorption processes as well as the ion-exchange process. There is uniform distribution of absorbate throughout the body of absorbent.
Key Differences between Absorption and Adsorption. The process of Absorption remains the same throughout the material whereas the Adsorption is a process that is determined by the concentration of the substances. Both phenomena along with ion exchange fall under.
Adsorption is the physical adherence of molecules or ions onto the surface of another compound. Absorption - process of one substance being absorbed by another. Chemisorption or chemical adsorption is adsorption in which the forces involved are valence forces of the same kind as those operating in the formation of chemical compounds.
Adsorption takes place on the surface of a substrate. The key difference between adsorption and desorption is that adsorption refers to the process by which some solids hold the molecules of a gas or liquid or solute as a thin film whereas desorption refers to the release of an adsorbed substance from a surface. The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or a solid resulting in a higher concentration of the molecules on the surface is called adsorption.
Adsorption and desorption are chemical processes that are opposite to each other. Heat is evolved during process. The primary difference is the physical interaction between the sample components and the mobile and stationary phases used.
Absorption is an endothermic process whereas Adsorption is an exothermic process. Difference Between Absorption and Adsorption The uniform distribution of the molecular species throughout the bulk is called Absorption. Difference between Adsorption and Partition Chromatography.
Therefore the primary difference between adsorption and absorption is that absorption is a bulk phenomenon which means that it happens throughout the body of the material whereas adsorption remains to be a surface phenomenon. In absorption the reaction rate is uniform whereas in adsorption the reaction rate is steady and gets equilibrium. The adsorbent surface retains excess concentration of the adsorbate.
It is bulk phenomenon. Adsorption - process of a thin film of the liquid or gas adhering to the surface of a solid substance. The difference between absorption and adsorption is straightforward.
The main difference between absorption and adsorption is that the process in which a fluid gets dissolved into a solid or liquid is known as absorption while when the atoms ions and molecules from one substance adhere to the substance in which they are meant to adsorb it is known as Adsorption. It is a surface phenomenon. A gas absorbed by a liquid.
This process is different from adsorption because in adsorption the atoms molecules or ions adhere onto the bulk surface whereas in absorption atoms molecules or ions enter into the bulk material. Adsorption is an exothermic process as the energy of the surface decreases as it leads to a reduction in residual forces of the surface. Adsorption is always exothermic whereas absorption is endothermic.
Absorption is a bulk phenomenon on the contrary Adsorption is a surface phenomenon. Chromatography is a laboratory technique that is utilized in the context of separation of mixtures. The foremost absorption and adsorption is absorption is the strategy whereby fluid is dissolved by each robust or liquid.
Describe the difference between absorption and adsorption. Absorption is an endothermic process as the energy is given from the outside of the surface and the overall energy of the absorbent increases after absorption. The major difference between adsorption and absorption is that one is a surface process and the other a bulk process.
The absorbent surface doe not retains excess concentration of the absorbate. Here the sample components physically adsorb stick to the stationary phase. Absorption is the process whereby a substance is taken in the bulk of another substance whereas adsorption is the process whereby adsorbate sticks on the surface of the adsorbent.
Absorption one substance enters the bulk or volume of another substance eg. The key difference between absorption and adsorption is that in absorption one substance.

Adsorption And Absorption What Is The Difference In Them Chemistry Topperlearning Com Ih01kxqq
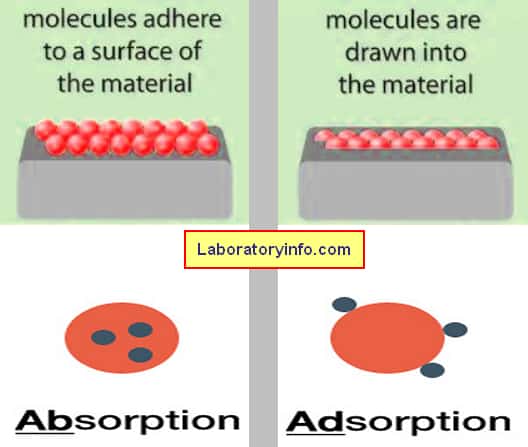
Difference Between Absorption And Adsorption Laboratoryinfo Com

Difference Between Absorption And Adsorption In Tabular Form Whatmaster
No comments for "Describe the Difference Between Absorption and Adsorption"
Post a Comment